Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


Aptar CSP Technologies – a leader in active packaging solutions that ensure product protection, enhance brand recognition and improve use experiences, and part of AptarGroup Inc – is presenting its Activ-Blister™ Solutions for the protection of moisture and oxygen-sensitive tablets and capsules, alongside FreeThink Technologies and its ASAPprime® technology for accelerated shelf-life determination. The combination is a “right the first time” approach to stability challenges and blister package design that virtually eliminates protracted testing and costly reformulations. The webinar, titled Rethinking Oral Solid Dose Packaging, will be conducted on March 28 at 11:00am EST.
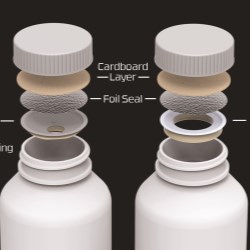
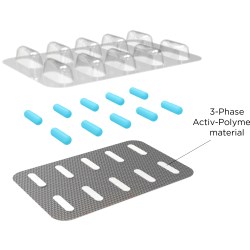
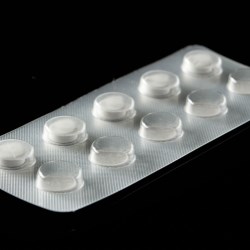
Activ-Blister solutions control the internal atmosphere of individual blister cavities, allowing for improved product performance and enhanced shelf-life. Using proprietary three phase polymer technology, engineered materials can absorb customized amounts of water vapor, oxygen, and/or volatile compounds, and can be produced in shapes and sizes to accommodate any tablet and capsule size. This innovative technology can be applied via heat-staking, without the use of adhesives, and added to existing packaging lines.
Working together with FreeThink Technologies, a leader in accelerated shelf-life determination, and utilizing their ASAPprime technology, a drug product’s moisture and oxygen sensitivity is determined using highly accelerated stability studies open to specified environmental conditions – such as temperature, relative humidity and oxygen level. With this analysis, modeling creates a theoretical blister design with Activ-Blister solutions to achieve desired shelf life.
Once the optimized blister and sorbent are determined, laboratory, clinical and stability study packaged product can be manufactured. This total process from Aptar CSP Technologies is called Xcelerate and provides expedited end-to-end expertise for packaging of sensitive oral solid doses, from R&D to commercial launch.
The combination of Activ-Blister solutions and ASAPprime technology is in response to the increased complexity of matching moisture- and oxygen-sensitive oral dose products to ideal packaging solutions, as well as to the ever-growing demand to expedite time to market. Tablets and capsules are today’s most popular drug delivery forms, and stability issues are only likely to increase with the development of more potent API’s, larger molecules and modified release profiles. Innovative and evolving dosage forms, such as chewable and disintegrating tablets, also face heightened stability challenges and problems of shelf life. Adding to all of this, global distribution demands in zone 4a/4b (hot/humid) are also increasing.
“Activ-Blister solutions and the Xcelerate complete packaging service offer improved stability, extend shelf-life and expedite time to market for sensitive oral solid dose products,” said Craig Voellmicke, Vice President, Business Development, Aptar CSP Technologies. “Together, they provide a valuable combination of effective drug protection and ease of adoption.”




.jpg)
.jpg)





.jpg)
.jpg)













.jpg)




.png)