Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


From stability challenge to commercial launch, Xcelerate expedites packaging process for medicines sensitive to moisture, oxygen and volatile compounds
Aptar CSP Technologies, a leader in material science and active packaging solutions that ensure product protection, extend shelf life, and improve user experiences and part of Aptar Group, Inc., announces the launch of a complete solution service offering focusing on de-risking development and insuring speed-to-market. The new Xcelerate Development Services (“Xcelerate”) optimizes the active packaging development process through end-to-end expert insight, resulting in expedited speed-to-market. Xcelerate was recently awarded the 2019 All-Star Innovator by Pharma Manufacturing Magazine.
Xcelerate combines Aptar CSP Technologies’ unique material science and active packaging solution and associated services with FreeThink Technologies’ leadership in accelerated predictive modeling in shelf-life determination. Utilizing FreeThink’s ASAPprime® technology, an optimized active packaging solution product is modeled and tested without the need for expensive, time-consuming line trials. The result is a “right the first time” approach that virtually eliminates repetitive design processes and reformulations caused by stability test failures.
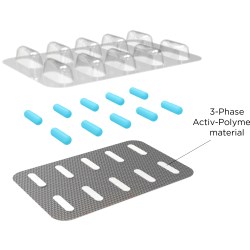
Aptar CSP Technologies offers a portfolio of services (research and development, clinical and stability studies, commercial planning and production, etc.) built around its proprietary 3-Phase Activ-Polymer™ technology platform, which is delivered in a variety of configurations:
• Activ-Blister™ solutions control the internal atmosphere of individual blister cavities. Applied via CSP Technologies’ patented heat-staking process without the use of adhesives, the technology accommodates any tablet and capsule size.
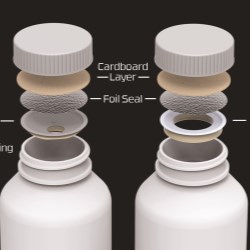
• For bottled medicines, Activ-Seal™ closures incorporate moisture and gas scavenging technology press-fit into an induction-sealed, tamper-evident screw cap.
• The Activ-Vial™ portfolio consists of one-piece, flip-top desiccated vials and desiccated bottles across a range of sizes.
• Activ-Film™ was developed to enhance transdermal drug delivery systems (TDDS), which provide controlled delivery of active substances through the skin.
Xcelerate reduces the entire active packaging design, testing and implementation process. The comprehensive, hands-on approach also includes capabilities to produce clinical trial and stability samples, regulatory support, consulting and testing during commercial implementation.
Aptar CSP Technologies launched Xcelerate in response to the increased complexity of matching moisture- and oxygen-sensitive medicines to ideal packaging solutions. As drug companies produce more potent APIs, larger molecules and modified release profiles, there is increased risk for stability issues associated with moisture, oxygen and volatile reactives. Innovative dosage forms such as chewable and disintegrating tablets also face significant shelf life challenges with respect to humidity sensitivity. These products may also face the added challenge of global distribution demands in Climate Zones 3/4b (hot/humid).
Xcelerate’s customized process determines a drug product’s moisture and oxygen sensitivity using highly accelerated stability studies open to specified environmental conditions such as temperature, relative humidity and oxygen level, then uses modeling to create theoretical package designs. Once the optimal packaging sorbent levels are determined, laboratory, clinical and stability study sample supplies can be prepared for confirmation or actual use.
“Our complete solution of material science driven active packaging solutions, and the newly launched Xcelerate Development Services offer improved stability, extended shelf-life and expedited time-to-market for sensitive medicines,” said Craig Voellmicke, Vice President, Business Development, Aptar CSP Technologies. “Together, they provide a valuable combination of effective drug protection and ease of adoption.”



.jpg)
.jpg)




.jpg)
.jpg)













.jpg)




.png)