Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


François Bidet, CSP's Director of Business Development for EMEA, sat down with us recently in order to review some of the company's latest offerings. Of special note is the company's upcoming Activ-Blister concept, which takes desiccant foils manufactured by the firm and applies them to blister packaging for pills and capsules.
François, what is the structure of CSP?
We belong to the Wendel Group, a large French investment firm with exceptionally diverse holdings. The group has investments in numerous sectors and products, including insurance, mobile telephone service, building materials, paint, industrial components, baking machinery, and packaging, of course.
As part of the group, CSP is a customer-focused global provider of custom polymer solutions and specialty protective packaging. We're the world’s leading manufacturer of plastic vials used for storing diabetes test strips, due to our patented technology for plastic desiccant vials.
We're a one-stop-shop and take pride in the fact that we can help our customers go from a simple concept through to delivery, taking into account all their needs and making sure we get the job done correctly according to their requirements.
What is the extent of CSP's operations?
We have specialized manufacturing plants in France and the USA, and a sales office in Asia as well as our corporate headquarters in Auburn, Alabama. Our operations produce more than a billion pieces of packaging every year, and much of that is in the health sector. We produce numerous patented packaging solutions for moisture- and/or oxygen-sensitive products. In fact, we have over 330 patents on file for our proprietary technologies.
We're also making great strides forward providing packaging solutions for the food sector, taking the know-how and experience we've garnered in the pharma space and applying it to that space. That's one of the reasons we acquired Maxwell Chase in 2016, they brought us capabilities to advance further in the food sector. They produce absorbent and non-absorbent packaging solutions, specifically for the food industry. In general, we create bespoke solutions and maintain a very high level of control over the specific projects on which we work.
What is your part in CSP's activities?
I'm the Director of Business Development for Europe, the Middle East, and Africa. I have a sales and marketing role where I develop strategic and long-lasting relationships with clients in order to ensure they receive the best service possible. I also market to prospective clients and let them know our capabilities so they can make informed decisions prior to engaging in large scale packaging development projects.
What sort of companies do you target?
I tend to focus on large scale brands in the pharma sector, international players that can genuinely benefit from the products and services we offer. We have a wide range of regulatory-compliant solutions in a variety of form factors, each of which can be tailored to meet customers’ specific needs and improve the consumer experience. So, our customers tend to be multinationals that have specific packaging needs. Companies like Roche, Merck, Abbott, Johnson & Johnson, and many others.
We also work with a lot of smaller companies, start-ups that are looking to market new concepts and that require specialized packaging items that only we can provide. One of the reasons our large and small customers come to us is the fact that we can do the whole packaging project, all the way through to the decoration, even labelling.
What are your best selling products?
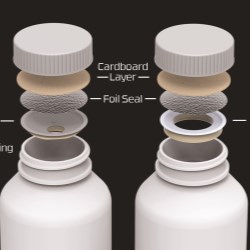
Our current best seller by far is our Activ-Vial line of products. The Activ-Vial is a range of products that are one-piece, flip-top vials or bottles with desiccants in a wide range of sizes. This line offers protection for drugs and medical devices against humidity. The attached lid cannot be lost or misplaced and seals to make the pack moisture-tight and leak proof. The patient that uses a pharma product in our packaging is completely assured of receiving a safe and protected product. Even if the pack remains open for five minutes, it only registers 5% humidity or less, it's a very effective piece of packaging thanks to our patented desiccant system.
And what new items are you releasing soon?
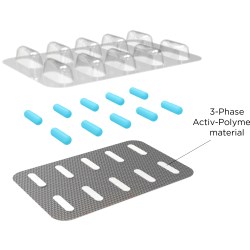
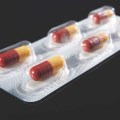
One of our biggest breakthroughs is in blisters. You have to understand that in Europe, blister packaging for pills and capsules is the standard. It's the safest, most widely used paradigm there is in the European market. Our new Activ-Blister line is dedicated to offering moisture control for individual pharma items, in a simple and recognized packaging concept that consumers trust. We put small pieces of desiccant film in the blister, and it goes a long way in keeping the medication efficacious. The new line is based on our existing line of Activ-Film items, except we put everything together for the client so they're ready to be used.
Where does the CSP team develop these new concepts?
We have a Global Innovation Centre in Paris. We have a great team of highly trained and experienced engineers and technicians that take client projects or marketing recommendations and develop packaging concepts. They make sure to satisfy the relevant requirements and make educated suggestions on improvements. Development of specific projects works in parallel with R&D efforts. We're always exploring new avenues, new ways to offer our customers better and more complete service.
Does this work take place in a clean room environment?
Our bi-injection and bi-extrusion lines satisfy all the regulations in effect for that sort of packaging, but they don't manufacture in a full clean room environment, it's unnecessary. Other products, the ones that include desiccants and aseptic packaging do, of course, occur in a clean room setting. We're very careful about producing products in the appropriate setting, especially because of the various regulations in Europe, the Americas, and Asia. We try and stay ahead of changing legislation by keeping our quality level beyond what's required.
What quality processes does CSP engage in?
We follow Six Sigma quality principles. The system defines a number of manufacturing and business processes that are designed to reduce occurrences of error to nearly negligible statistics. The primary goal is to reach large scale manufacturing where there are only three or four defective items produced per million. Our lines are rigidly calibrated, controlled, and maintained. We also have several quality checks on the line, so we're sure to catch sub-standard items prior to shipping them. Initially, we garnered our Six Sigma certification because a large customer of ours was operating under those principles and required we adopt the method if we wished to be a partner. It turned out to be a great idea, we've really come to rely on the processes and our lines are some of the most error-free there are.
- Activ-Film, Activ-Blister, and Activ-Vial are trademarks of CSP Technologies, Inc., AL.




.jpg)
.jpg)





.jpg)
.jpg)












.jpg)




.png)