If this is your company, CONTACT US to activate Packbase™ software to build your portal.


The core business of Verona-based company, Discos, is the manufacture of single dose products; whether standard or made-to-measure strips, for the cosmetic, parapharmaceutical and food industries. Founded by Roberto Speranza and Eleonora Savino in 1988, the company's Oppeano factory covers 6000m2 of which 1200m2 is used for offices and laboratories.
After an initial consolidation period in the packaging sector, since 2001 the company has dedicated production to single dose and made-to-measure strips.
An interview with Laura Speranza, Vice-Administrator and daughter of the company founders:
Dr. Speranza, Discos' operations involve the single dose concept, but in a very innovative way. Tell us more.
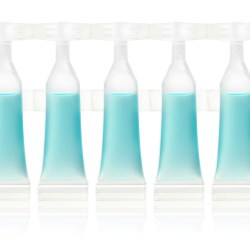
Yes, our single doses are a blend of technology and elegance - just a few millilitres can hold parapharmaceutical, cosmetic or food formulas. Our service covers the manufacturing and personalization of the single dose vials to their filling in a contamination-controlled environment, before the product is placed into secondary packaging and prepared in boxes on pallets for later handling in the goods warehouse.
Do you offer a made-to-measure version as well as the standard models?
The made-to-measure product arose out of the idea of a sealed single dose, with a system of hooks enabling strips of made-to-measure single doses, that is, containing either higher or lower quantities than the standard 5 vial version. It is a versatile and functional solution, which is available in 4ml and 6ml versions, manufactured in PP or PE.
Along with using variable numbers of strips, we can fill them with different products, based on type or viscosity, and we can also differentiate them with different sealing systems depending on the amount of product to be contained.
What other services do you offer to your customers?
In order to respond to customers' demands and increase our competitiveness in the market, we also fill jars, bottles and tubes to complement our main activity. This allows us to supply a customer requiring a range of products with different formulas and packaging, for example; and in Discos, they find a packaging partner that can meet all their needs.
Does all the manufacturing occur in-house?
The technological infrastructure of our Oppeano headquarters allows us to handle the entire production process. We have a ISO 8 certified room and three single dose filling lines - two for cosmetic products and one for medical devices with the EC mark.
We also have two tampographic printing devices to personalize the single dose vials here, with 2 colours for the front and 1 for the back; a semi-automatic filling machine; a filling/sealing line for bottles from 15ml up to 1000ml; three manual fillers for filling 10 to 350ml; a manual labeller for round bottles; an automatic labeller for round bottles; a glass vial filling machine with 2 sealing flames with a productivity of 2000 vials/hour; three ink injection machines on the three semi-automatic lines; 2 manual punches for the batch number; and a heat wrapping machine for medium and small batches.
The single doses are personalized using a tampographic print on one or both sides in several colours, and the batch and expiration date is printed by ink injection. Manufacturing, such as for nasal fluids and eye drops, is undertaken in contamination-controlled environments in accordance with the EC mark for medical devices.
Discos also uses a quality control system during the process and each production stage is recorded in modules that respect the set manufacturing parameters in accordance with the ISO 9001:2008 and ISO 13485:2012 standards. The production potential is around 100/10,000 parts/day for jars and bottles and around 25,000 for single dose strips. The finished product is packaged in the corresponding boxes on pallets, ready for shipment. Each box is labelled with the product contained, the batch, the expiration date and the number of objects it includes, whilst the pallets come with a packing list of the exact contents.
Discos operates primarily in the Italian market with approximately 20% of the company's production distributed across the European Union. Managed by a young and active team, Discos offers a made-to-measure service for cosmetic, parapharmaceutical and food products, focused on meeting customer needs through constant constructive dialogue from production and filling which continues until the product is boxed in pallets for appropriate handling upon arrival at the goods warehouse.