Public
Gerresheimer Brochures
Gerresheimer Gallery
Gerresheimer Locations
Gerresheimer News
Gerresheimer Product Catalog
Gerresheimer Publications "Update"
Gerresheimer Videos
Subsidiaries
Sensile Medical
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


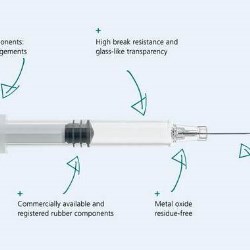
Gerresheimer is now manufacturing the first Gx RTF ClearJect plastic syringe made of COP in Europe
COP does not leach any metal ions into the liquid pharmaceutical drug. The entire syringe, including the needle overmould, is produced in one single step and the product is also tungsten and adhesive-free. COP has a high pH tolerance and, unlike glass, there is no change in pH value over time during storage. Another key argument in favor of the Gx RTF ClearJect syringe is excellent user safety. COP is extremely inert and break resistant, so it is an excellent choice as a packaging material for sensitive or toxic active ingredients. High elasticity in comparison to similar materials also enhances the Dupont impact strength of COP syringes.The use of a high-viscosity silicon oil to coat the inside of the syringe barrel reduces the level of syringe content contamination with silicon particles, and the integration of standardized closure components also offers the pharmaceutical manufacturer flexibility. The application of a concept to create the entire innovative COP syringe out of standard components ensures its cost-efficiency. It has standard needles, plungers, plunger heads, backstops and closures.
COP is an interesting alternative to the traditional glass syringe as a result of the new demands on primary packaging posed by innovative active ingredients. Oncological drugs can be extremely aggressive, so syringe break resistance is an important criteria in the selection of their primary packaging. Innovative biotech drugs, on the other hand, are often administered in tiny dosages and many of them are very expensive. With this type of drug, it is important to rule out any interaction between the syringe material and its content. COP meets all these requirements.
Presentation on the use of tungsten in the glass syringe moulding process
“A few liquid dose pharmaceuticals react to the traces of tungsten that are left behind during the drilling part of the syringe manufacturing process,” said Bernd Zeiss, Biologist and Technical Support Manager at Gerresheimer Medical Systems in Bünde. He explains why this is the case and talks about low-tungsten and tungsten-free syringe alternatives for sensitive applications in his presentation.
Presentation on DUMA Twist-Off Protect – the new generation
“Pharmaceutical drugs are often sensitive to moisture vapor and oxygen. As a result, they need more effective protection from suitable packaging,” said Dr. Wolfgang Dirk, Product Management and Innovation at Gerresheimer. “That’s why we’ve extended our established range of Duma-family products to include an additional model with improved barrier properties for enhanced air-tightness and more reliable content protection.” He will be explaining the key aspects, potential pitfalls and applications for multilayer containers and their closures.
Ampoules and vials are top sellers
Vials are one of Gerresheimer’s most popular and top-selling products. There is high demand for them because they are one of the most frequently used pharmaceutical packaging products in the world. Gerresheimer manufactures them in the USA, Asia and Europe with filling volumes of between 1 and 50 ml.



.jpg)
.jpg)
.jpg)













.jpg)