If this is your company, CONTACT US to activate Packbase™ software to build your portal.
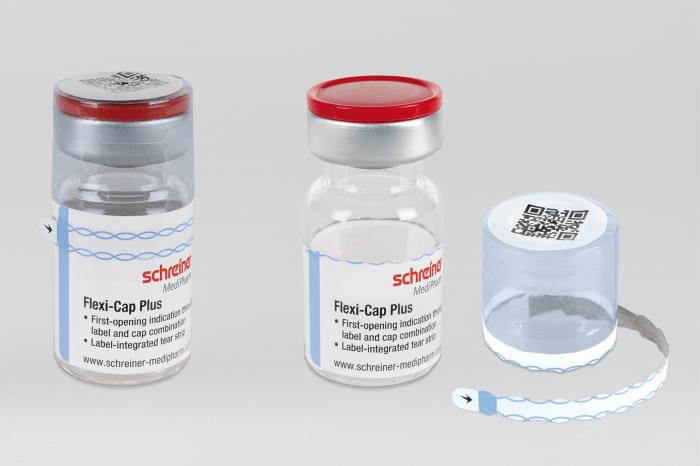

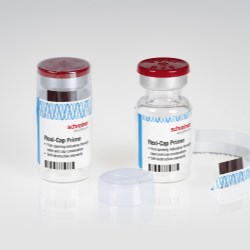
Schreiner MediPharm, a Germany-based global provider of specialty pharmaceutical labeling solutions for over 60 years, has introduced the next generation of its popular Flexi-Cap, an innovative security solution that irreversibly indicates the first opening of primary containers to prevent their illegal reuse with counterfeit substances. The new Flexi-Cap Plus features a tear strip running through the label as an integrated element. The result is enhanced protection against tampering, as the label is destroyed upon initial opening and can no longer be reused as an alleged original.
Illegal reuse of empty medicine containers with original labels poses a major problem to pharmaceutical manufacturers. So-called dumpster divers, for instance, sift through rubbish for used original containers and fill them with ineffective or harmful counterfeit substances. The alleged originals are subsequently put on the market without revealing any signs of tampering at first glance. To counteract these illegal practices, the innovative Flexi-Cap security concept by Schreiner MediPharm is based on the combination of a film cap with a label for clear and irreversible first-opening indication.
“The enhancement introduced with the new Flexi-Cap Plus is that the tear strip is integrated directly in the label rather than run through the film cap,“ explains Gene Dul, President of Schreiner MediPharm U.S. “When the tear strip is opened, it automatically destroys the label and makes it impossible to illegally reuse the container with this original label.”
By adding other overt and covert security technologies, such as holograms, color-shifting inks, void effects or security pigments, further hurdles to tampering can be raised. Flexi-Cap Plus can be flexibly customized to accommodate various glass container types, shapes and sizes, and is easily integrated into a pharmaceutical manufacturer’s existing brand design. Particularly useful for small containers, the lid of the film cap also offers additional space that can be used for imprinting barcodes or integrating NFC chips for interactive applications.
The label construction is applied without heat and thus suitable for temperature-sensitive medicines. According to Mr. Dul: “Flexi-Cap Plus provides end users in healthcare settings with an efficient, intuitive security solution that makes it easy to check first opening. The solution is another step forward in protecting patients against the administration of ineffective or harmful medications.”
Schreiner MediPharm will present the new Flexi-Cap Plus, as well as a variety of additional labeling solutions, at InnoPack as part of CPhI Worldwide in Barcelona from October 4–6.


.jpg)




















.jpg)