If this is your company, CONTACT US to activate Packbase™ software to build your portal.

For a multinational pharmaceutical corporation, Schreiner MediPharm developed a smart blister pack for digital patient compliance monitoring to enhance medication adherence by clinical trial participants. Schreiner MediPharm implemented the smart packaging solution together with the Dutch technology company ECCT (Experts in Communications and Connectivity Technology). Employment of this electronic tool to manage and track processes during clinical trials marks a milestone for the pharmaceutical manufacturer.
Clinical trials are normally conducted on an international scale and require high accuracy, reliable workflow, efficiency, speed and flexibility. Conventional, non-automated processes are frequently error-prone. Medication adherence by participating patients, however, is a key factor for the successful outcome of clinical trials, but often difficult to track. For instance, based on a rule of thumb, 20 percent of patients do not adhere to the therapy. Accordingly, an increase of the patient population by 60 percent is necessary to compensate for the lack of clarity in the results.
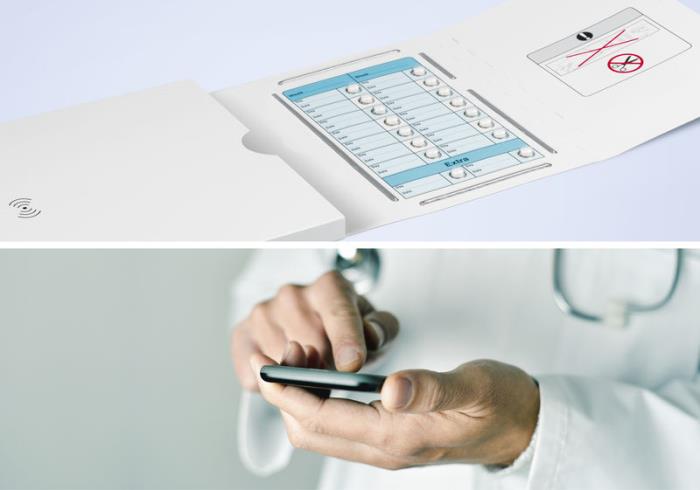
To address these challenges, Schreiner MediPharm, in cooperation with ECCT and the pharmaceutical corporation, developed a smart packaging solution for patient compliance monitoring: Pressing a tablet out of the blister pack generates data in real time such as the type of medication, the time of extraction and the respective cavity. This information is automatically stored in the smart package and transmitted to a database via a smartphone app or reader. Compliance of the respective patient is thereby tracked. Additionally, it is possible to send the patient a reminder to take the medication, to adjust the dose and to assist trial participants with interactive communication between the physician and patient in the interest of medication adherence.
The smart packaging solution includes printed electronics without impacting the packaging design. A database platform enables diverse data transfers and analyses. Schreiner MediPharm supplies the required expertise in innovative printing technology and ECCT the smart sensors.
The utilization of the digital patient compliance monitoring tool significantly reduces the manual documentation for the pharmaceutical corporation. Complex therapies and trial processes can be adapted with greater flexibility, the delinquency rate due to proven non-compliance reduced and data quality optimized. In addition, the smart packaging solution can shorten the overall trial period and accelerate the approval process for new medicines.


.jpg)



















.jpg)