If this is your company, CONTACT US to activate Packbase™ software to build your portal.


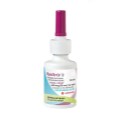
Aptar Pharma, a global solution provider of innovative and proven aerosol, injection and spray delivery systems for biotech, healthcare and pharma products, will unveil its latest innovation, the eDose Counter for metered dose inhalers (MDIs), at the RDD scientific conference in Phoenix, Arizona from April 18-21, 2016.
Dose Counters improve Asthma and C.O.P.D. patient adherence Asthma and COPD patient adherence is a major health and economic challenge. Several studies report very poor adherence to asthma medication regimens, with measured rates of non-adherence ranging from 30 to 70 percent.
In the FDA’s Integration of Dose-Counting Mechanisms into MDI Drug Products guidance for industry, dated March 2003, it is recommended that drug manufacturers integrate a dose-counting system into any new MDI drug products marketed in the US.
Patients appreciate dose counters because they are convenient and improve safety by allowing them to identify the number of doses of medication left in their inhalers and to avoid running out unexpectedly. The global inhalation market has followed the US trend and today multiple MDI drug products with some form of dose-tracking system are marketed outside of the US - including Skye Pharma’s Flutiform® equipped with Aptar Pharma’s Landmak® dose indicator.
Electronic Dose Counters offer enhanced performance
Currently, marketed MDI dose indicators and counters are based on mechanical technology. Although this technology offers great opportunities to design simple and cost-effective devices, it does not always meet the expectations of users, regulators and other stakeholders due to poor legibility of the numbers, its effect on MDI user-handling and pharma performance, and the lack of robustness impacting safety (e.g. miscounting events).
Electronic components offer almost unlimited possibilities to display large, clear and legible counter digits required for a wide range of user age segments and medical conditions. In addition, this technology allows for the incorporation of visual MDI priming reminders and feedback on use as well as end-of-product-life warnings. Thanks to the integration of electronic components, these systems not only count doses, but mark a move towards comprehensive connected inhaler solutions.
Aptar Pharma’s eDose Counter for MDIs integrates a unique sensing technology MDIs are complex systems that integrate several components such as the metering valve, the canister and the actuator. These components must be carefully designed and manufactured to ensure they work together effectively. This is particularly true for the valve and the dose counter. As the world leading supplier of MDI valves, Aptar Pharma’s expertise and experience enables a smooth integration of all MDI components, including the dose counter.
Our patented eDose Counter for MDIs is designed to be easy-to-use and reliable, and contributes to patient compliance. Our unique sensing technology offers direct detection of the spray which eliminates risks of miscounting. The sensing technology is versatile and compatible with any metering valve design. In addition, our eDose Counter for MDIs includes inhaler priming and medication reminders-to-use and end-of-product-life warnings (flashing digits) while remaining cost-effective.
“Aptar Pharma is very pleased to launch our novel electronic Dose Counter for MDIs. We believe the combination of Aptar Pharma’s expertise in MDI valves and dose counters with our experience in electronically-assisted drug delivery devices creates a compelling offer to meet the needs of Asthma & CODP patients”, commented Jean-Marc Pardonge, President of Aptar Pharma Prescription Division.


.jpg)



.jpg)
.png)
.jpg)


 copy.jpg)


.jpg)
.jpg)























.jpg)


