If this is your company, CONTACT US to activate Packbase™ software to build your portal.
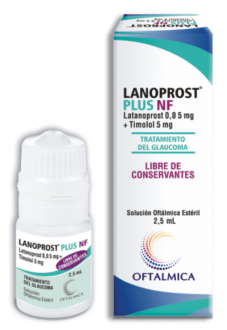

Our preservative-free multidose eyedropper has been launched in Paraguay with different formulations (branded OFTALMICA):
- Latanoprost
- Latanoprost + Timolol
- Dorzolamide + Timolol
The use of preservatives can cause allergies or ocular irritation, and some can even cause a toxic response, damaging patients’ eyes[*]. These reactions are even more pronounced when the patient uses combination therapies.
For this reason, patients under chronic treatment, like glaucoma, will appreciate the unpreserved formulations delivered with the multidose Novelia® system.
User-friendly and intuitive, Novelia contributes to improve patients’ life, allowing the delivery of safe unpreserved eye drops and improving the patients’ adherence to treatments.
Novelia has also been commercialized in Paraguay for artificial tears, decongestants and anti-allergy applications.
[*] Report of the International Dry Eye Workshop. Ocul Surf 2007; 5[2]; 65-204.