If this is your company, CONTACT US to activate Packbase™ software to build your portal.


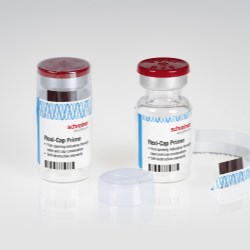
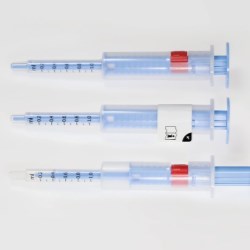
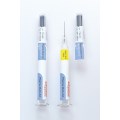
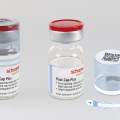
New Flexi-Cap for clinical trial supplies combines anti-counterfeit protection with reliable binding for transparent containers; Integrated Booklet-Label offers ample space for product info in different languages.
Schreiner MediPharm, a Germany-based global provider of specialty pharmaceutical labeling solutions for over 65 years, has a new addition to its Flexi-Cap product family: Flexi-Cap for Clinical Trial Supplies (CTS).
Schreiner MediPharm’s Flexi-Cap solutions irreversibly indicate first opening of primary containers, thus preventing illegal filling and reuse of empty containers with counterfeit substances. The new Flexi-Cap for clinical trials adds reliable blinding of transparent containers to this first-opening protection, accomplished through the use of opaque-printed film caps that prevent investigational products from being distinguished from one another.
The new Flexi-Cap for Clinical Trial Supplies consists of a label and two opaque-printed film caps that completely wrap around the container. While one cap encloses a container’s lid and upper portion, the second cap protects its bottom and lower sections. In this way, the solution ensures reliable blinding of transparent containers, combined with irreversible first-opening protection due to a tear strip integrated into the upper cap.
The need for this combination label concept arose from the requirement that product candidates in clinical trials be reliably blinded in order to achieve optimum test results. Since any visual differences between the active drug and the placebo – including variations in color – must not be discernible to trial subjects, labels need to meet special requirements when transparent containers are used. The new Flexi-Cap version also protects clinical trial supplies against tampering, and provides space for extensive product descriptions in diverse languages – a prerequisite for clinical trials to be efficiently and flexibly conducted in an international context.
Applied without heat, the solution is suitable for temperature-sensitive medicines. Flexi-Cap for Clinical Trial Supplies can be adapted to glass containers of various types, shapes and sizes and also protects against glass breakage. Various colors with high opacity may be used for the caps, and the lid of the film cap offers additional space that can be imprinted with codes or used for integration of NFC chips for interactive applications.
“Schreiner MediPharm is proud of the newest member of our Flexi-Cap family, which combines first-opening protection with reliable blinding in a unique fashion,” said Gene Dul, President of Schreiner MediPharm U.S. “This innovation speaks directly to the critical need for investigational products used in clinical trials to be both truly secure and indistinguishable from each other in order to assure successful research studies.”



.jpg)



















.jpg)