If this is your company, CONTACT US to activate Packbase™ software to build your portal.


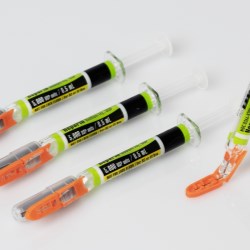
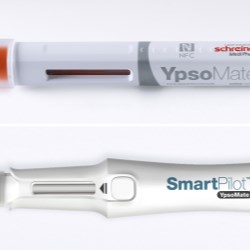
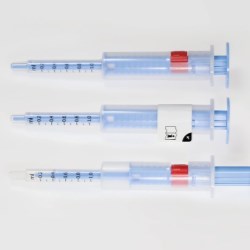
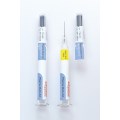
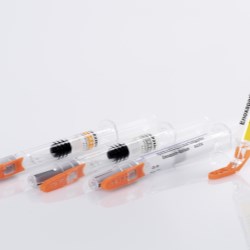
Schreiner MediPharm, a Germany-based global provider of specialty pharmaceutical labeling solutions, has collaborated with B. Braun Medical Inc., located in Bethlehem, PA, who recently launched its prefilled syringe of Heparin Sodium Injection, USP utilizing Schreiner MediPharm’s label- integrated Needle-Trap system to the U.S. market.
According to B. Braun, this is the first prefilled heparin syringe with an integrated needle protection device approved by the U.S. Food and Drug Administration (FDA).
In the United States, syringes should be used with safety devices to protect healthcare staff against the risk of needlestick injuries. B. Braun Medical was looking for an efficient and safe needle protection solution for its prefilled Heparin Sodium Injection and found it in Needle-Trap from Schreiner MediPharm.
The Needle-Trap system is an internationally established solution that complies with the U.S. NIOSH requirements for safe instruments and has been awarded 510(k) Pre- Market Notification by the FDA for marketing in the United States.
“Clinical staff benefit from reliable protection against needlestick injuries because Needle-Trap’s activation is easy and irreversible. It is intuitive to use and requires no change in injection technique,” says Gene Dul, President of Schreiner MediPharm U.S.
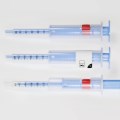
Needle-Trap is the only label-based needle protection system on the market. The plastic needle trap is an integral component of the marking label and serves to secure the needle after the injection has been performed.
Due to its special construction, Needle- Trap can easily and cost-efficiently be integrated into existing pharmaceutical production processes. It requires only minor modifications of the application systems, no changes to secondary packaging, and minimal space during shipping, storage and disposal.
“Our products are designed to increase patient and clinician safety, while reducing medication errors and improving dosage accuracy and workflows. Obviously, efficient and economical manufacturing are equally important. Collaborating with Schreiner MediPharm allowed us to meet those needs,” says Leigh Nickens, Director of Marketing, Fluid Therapy and Injectable Drugs at B. Braun.



.jpg)



















.jpg)