If this is your company, CONTACT US to activate Packbase™ software to build your portal.
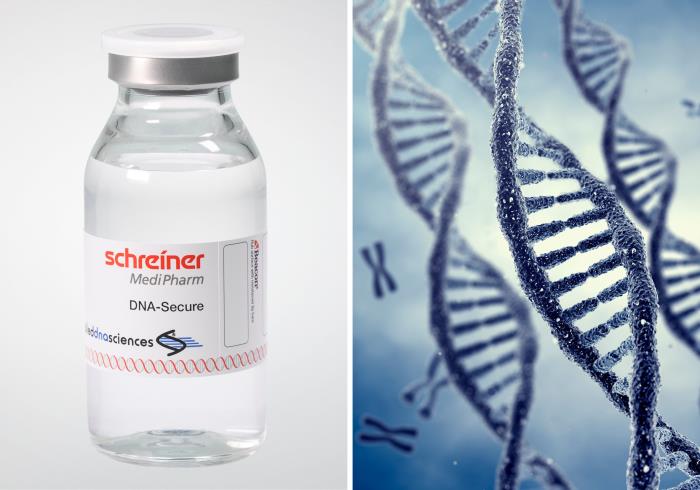

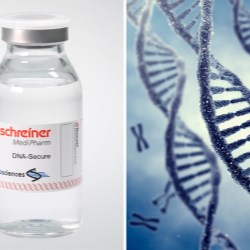
Schreiner MediPharm, a Germany-based global provider of specialty pharmaceutical labeling solutions, now offers a new forensic authentication feature for pharmaceutical labels in cooperation with Applied DNA Sciences. SigNature® DNA is a high-security feature based on DNA markers with which pharmaceutical manufacturers can protect products against counterfeiting, and patients against potential health risks. DNA markers are deemed to be impossible to counterfeit and recognized as forensic authentication evidence in courts of law.
A secure supply chain is indispensable in the pharmaceutical industry. If counterfeit medicines are put into circulation this may result in claims for damages by affected patients. Pharmaceutical manufacturers are able to counteract such illegal practices by providing their original products with forensic anti-counterfeiting features such as DNA markers.
Using conventional printing techniques, Schreiner MediPharm flexibly and invisibly integrates this high-security technology from Applied DNA Sciences into existing label designs.
Robust and counterfeit-proof, DNA molecular tags are covert authentication features based on uniquely modified, encrypted DNA sequences. Various multi-level methods to verify the authentication feature along the supply chain are available to informed experts: For example, Beacon® technology enables fast, reliable on-site verification by means of a decryptant liquid and UV lamp. Specialized mobile devices also may be used to authenticate the SigNature DNA molecular tags. An extensive forensic DNA analysis by a laboratory provides results that qualify as admissible evidence in courts of law.
With the innovative SigNature DNA marker, Schreiner MediPharm expands its portfolio of multi-level security systems and customized solutions for supply chain integrity.
“The integration of SigNature DNA in combination with Beacon® in our functional pharma labels will set a new security standard in the pharmaceutical industry," summarizes Nadine Lampka, Product Manager Pharma-Security at Schreiner MediPharm. "DNA-Secure is extremely robust, flexible to integrate and allows both a quick in-field test and an ultimate forensic proof in the lab. It provides excellent value for the investment, and complements our portfolio of integrated authentication features on the high-security end."
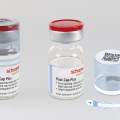
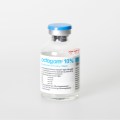
Caption: With the DNA markers integrated in pharma labels, Schreiner MediPharm and Applied DNA Sciences offer forensic proof of authenticity.


.jpg)




















.jpg)