If this is your company, CONTACT US to activate Packbase™ software to build your portal.
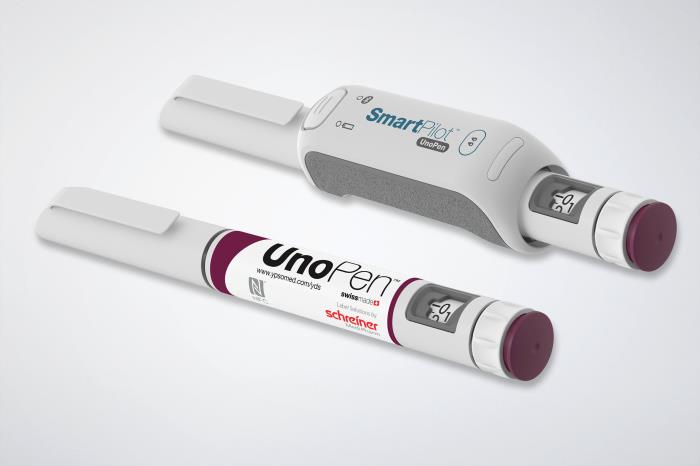

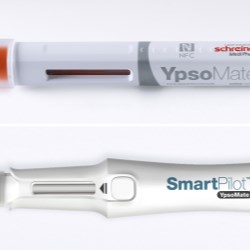
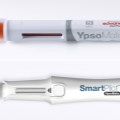
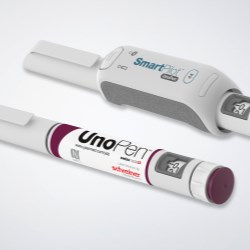
Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, has developed an NFC-Label for Ypsomed’s UnoPen, helping patients in combination with the SmartPilot add-on to properly utilize the device and adhere to therapy plans.
Diabetes patients depending on lifelong insulin treatment must administer the vital medication to themselves using insulin pumps, syringes or pens. Ypsomed offers UnoPen, a disposable pen with variable dose setting, for this and other multidose therapies.
Schreiner MediPharm’s NFC-Label serves as a communication interface between the injector and an electronic pen add-on called SmartPilot. Combined, the solution comprises a smart device that interactively supports patients in using the pen, and helps them adhere to therapy regimens.
Schreiner MediPharm designed the label with an integrated NFC chip precisely for the combination of the UnoPen and the SmartPilot. With the NFC-Label, the drug can automatically be identified, authenticated and checked in terms of its expiration date.
Via the smart device, the injection’s time, date and dosage are tracked and transmitted to the patient’s related smartphone app via Bluetooth. Patients are interactively guided through the injection process, assisted in correctly using the pen in real time, and informed about inconsistencies such as deviations from the therapy plan or accidental attempts to inject themselves twice.
Also integrated is a temperature monitoring feature that issues a warning in the event a pen is exposed to critical temperatures while being used or stored. Sensor technology installed in the SmartPilot that digitally connects the UnoPen enables this function. It is activated simply by attaching the smart device to the pen without covering the cap, the dose scaling mechanism and the button that triggers the injection. The device remains on the pen throughout the injection process.
Prior to introducing this latest solution, Schreiner MediPharm and Ypsomed were development partners in the SmartPilot application project for the YpsoMate autoinjector:
“We selected Schreiner MediPharm as cooperation partner again to offer pharma customers an optimal combination of connected device and smart NFC-Label,” says Andreas Schneider, Innovation & Business Development Director at Ypsomed.
The narrow radius of the pen and the small tolerances of the attached device posed particular challenges this time. Another key objective was automated processing, because the label-integrated NFC chip has to reliably function from production through patient use. Schreiner MediPharm utilizes a special design ensuring end-to-end functionality.



.jpg)




















.jpg)