If this is your company, CONTACT US to activate Packbase™ software to build your portal.


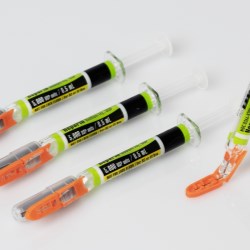
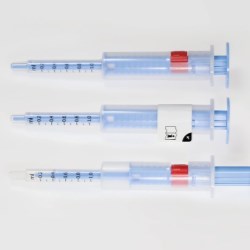
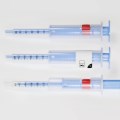
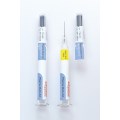
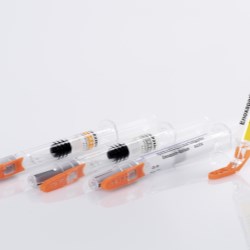
Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, has equipped prefilled enoxaparin-sodium syringes from Kingfriend China, a leading provider of heparin and heparin products, with its innovative Needle-Trap safety solution.
The move marks the first application of a China-based product for Schreiner MediPharm’s groundbreaking needlestick prevention system. The world’s only label-based needle protection solution, Needle-Trap has safeguarded more than 1.2 billion syringes in many international markets since its introduction in 2009.
Kingfriend offers its anticoagulant medication for thrombosis prevention as an enoxaparin-sodium solution in prefilled syringes. For its European market launch, the company was looking for a cost-efficient needle protection solution that meets all legal requirements.
Based in Nanking, the pharmaceutical company found its ideal solution with Schreiner MediPharm’s Needle-Trap. The needle protection label with an integrated trap marks the syringe and helps protect healthcare staff against needlestick injuries. Needle-Trap meets requirements of EU Directive 2010/32/EU for protection against injuries, as well as DIN EN ISO 23908 for protection against sharps.
Kingfriend was especially impressed by Needle-Trap’s design. The trap component is directly connected to the label, and features a particularly compact construction. As a result, the heparin syringes’ secondary packaging does not need to be modified, saving both warehousing and logistics costs. In addition, the economical solution requires only minimal production process adaptations.
The prefilled enoxaparin-sodium syringe with Needle-Trap was launched in Germany in the fall of 2020; the product is marketed via partner company Venipharm.


.jpg)




















.jpg)