Public
SGD Pharma Catalog
SGD Pharma Documentation
SGD Pharma Locations
SGD Pharma News
SGD Pharma Videos
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


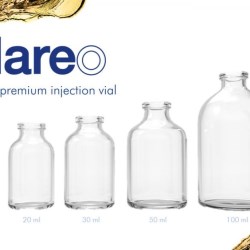
Worldwide glass primary packaging supplier SGD Pharma has announced the introduction of its 100H* ml Ready-to-Use (RTU) Type I molded glass vials for aseptic fill/finish of parenteral drug products.
The addition of 100 ml molded glass vials to the Sterinity platform is pioneering, with SGD Pharma being the first global molded glass manufacturing company to offer this size of solution in the RTU market. The 100 ml vials, available in both clear and amber molded glass, are powered by the Stevanato Group’s EZ-fill® platform. This allows global companies to accelerate the time-to-market of their high-value parenteral drugs with an RTU primary packaging solution suitable for large volumes.
The chemical resistance of molded glass makes the SGD Pharma 100 ml ISO version an optimum solution for organizations using vials for different injectable applications - the chemical inertness of molded glass protects drug stability, even for cytotoxic oncological drugs. The 100 ml vial in Stevanato Group’s EZ-fill® tray has a range of benefits including no glass-to-glass contact, which reduces particle generation to protect patient safety. This also has the benefit of eliminating scratches on the vials, supporting the visual inspection of the filled vial and reducing the possibility of rejection at quality control.
The tray is covered with two SteriBags, which minimizes the risk of contamination to the aseptic area. This packaging system guarantees five years of sterility while also protecting the physical integrity of the vial. SGD Pharma designed the 100 ml standard ISO 20 version to be easily integrated into existing manufacturing processes with minimal changes.
Alexander Bautista, Product Manager Sterinity Platform, said: “SGD Pharma is the sole player in the market that is currently offering 100 ml molded glass vials in SG® EZ-fill® trays and it’s important for us to be at the forefront of this innovation. Currently, 100 ml represents the boundary between small and large parenteral vials and by introducing sterile empty vials in this size, we can offer drug manufacturers and pharmacists an alternative option.
“The mechanical resistance of molded glass makes this large vial size a first-choice solution for drug manufacturers. The new vials, available immediately and in flexible quantities, are mechanically robust and can be transported globally, with a minimum order of one box for those who only need small quantities. Innovation is at the heart of SGD Pharma and this 100 ml vial launch highlights our commitment to supporting our customers and their products as we drive the market in the direction of having additional alternatives for primary packaging solutions.”
SGD Pharma was the first global glass manufacturer to offer RTU molded glass vials for injectable drugs. Its long-serving partnership with Stevanato Group has enabled the company to introduce 100 ml molded glass vials powered by EZ-fill® – an industry-recognized secondary packaging platform that supports end-to-end RTU processes. Upfront washing, depyrogenation, and sterilization steps are taken care of, with RTU Type I molded glass vials arriving in trays or in nest & tubs ready for manual, semi-automatic, or automating fill/finish, allowing drug manufacturers to focus on core business activities to expedite product launches.
The Sterinity platform includes ISO 20 vials in tray 50 ml and 100 ml and EasyLyo vials in tray 20 ml, 25 ml, 50 ml. EasyLyo 20 ml is available in nest & tub. All vials are available in clear and amber versions. EasyLyo provides stronger packaging that is more resistant and suitable to extreme conditions encountered during the lyophilization process. EasyLyo is a range of Type I glass vials that combines the strength of molded glass with superior cosmetic quality and weight reduction.
* ISO Standard 8362 designates molded glass vials with an H and tubing glass vials with an R