Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.
.jpg)

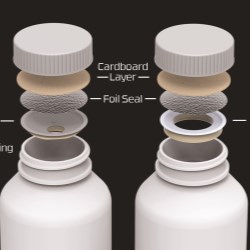
CSP Technologies, Inc. – a leader in designing and producing packaging solutions that ensure product protection, enhance brand recognition and improve consumer experiences – is expanding its Auburn, Alabama manufacturing and warehousing capacity by 110,000 square feet. Construction of the new structure, located adjacent to the existing headquarters, is currently underway.
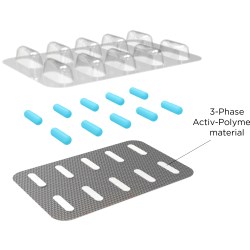
The expansion is necessary due to the recent growth in the areas of healthcare and food safety, and follows the commissioning of a new thermoforming line earlier in 2017. The thermoforming operations will be the vehicle to deliver an upcoming portfolio of antimicrobial products for food safety.
During this same timeframe, the company added clean room manufacturing and high cavitation stack cube molding with in-mold process capabilities to increase manufacturing efficiency at its plant in Neiderbronn-les-Bains, France. Both the US and France operations will be ISO 13485:2016 certified for medical device manufacturing and assembly. The clean room installation also provides the necessary infrastructure as CSP prepares to manufacture dry powder inhalers and other medical devices in the near future.
Over the last three years, CSP has greatly increased its global in-house design, engineering, material science and project management capabilities, providing a “one stop shop” from concept to commercialization. To its benefit, CSP Technologies has developed a built-in pipeline of excellent designers, engineers, and scientists by partnering with both Georgia Tech and Auburn Universities. This strong relationship has fostered internships into full-time opportunities at all disciplines.
These additional resources have resulted in a variety of new projects now nearing commercialization. The new infrastructure is anticipated to increase global production volume by as much as 25% in the areas of plastic processing – including injection molding, thermoforming, and product assembly and filling.
“CSP Technologies is looking forward to expanding our global manufacturing and warehousing capacity with great pride, as it allows us to further broaden our portfolio of innovative products and packaging solutions, particularly to address recent growth in probiotics,” said John Belfance, CEO of CSP Technologies. “With six worldwide locations, and more than one billion products manufactured annually, we will have the capability to provide even more customers with the competitive edge CSP provides.”




.jpg)
.jpg)





.jpg)













.jpg)




.png)