If this is your company, CONTACT US to activate Packbase™ software to build your portal.

Directive 2011/62 lays down rules to ensure at all times the authentication of medicinal products and their monitoring throughout the supply chain, which entails a series of measures to be applied by the different actors involved in the production and marketing of medicines.
During the last few months, we have been able to see how various bodies are being set up in Spain to control and set guidelines, given that major structural changes are required throughout the distribution and manufacturing chain.
Traceability is the main concern to ensure the quality and safety of medicines for patients and users. In addition to a system that revolves around identification software, solutions are required to avoid improper physical manipulation of medicines.
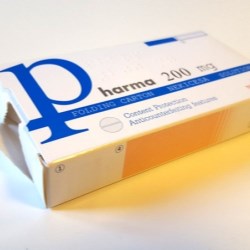
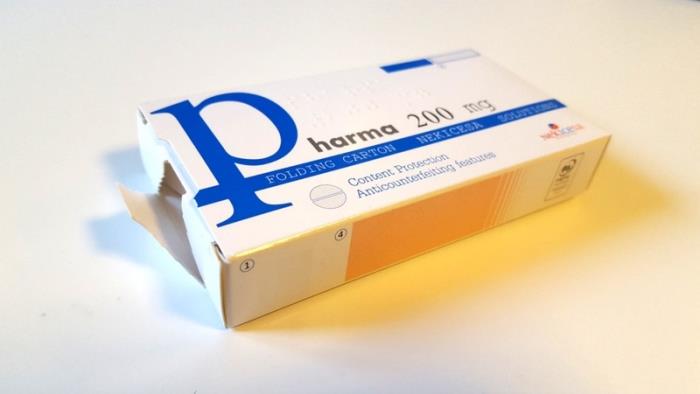
“Tamper evidence” devices are presented as one of the mandatory applications in folding cartons. These are solutions known also as solutions of inviolability, which allow to confirm if a package has been previously opened. In this way, it is reliably guaranteed that the content has not been adulterated or manipulated, for the tranquility of consumers and their safety.
This type of labeling acts as a security seal by placing it on the openings of the case. When opening the cartons, the manipulation necessarily becomes evident in a tangible manner, making it possible to guarantee authenticity of the product.
At Nekicesa, we choose different solutions based on the structure of the case or additional material (labels) that are applied on the closure of the folding cartons. In the first case, no additional material is required. In the second case, the labels imply an additional investment, both in material and in equipment in the packaging lines.
Regarding cartonsincorporating Tamper Evidence in the structural design of the case, we have two types:
- With the possibility of closing afterwards. In this case, it’s possible to re-close the packing afterwards, since the design of the folding cartons incorporates a closure flap that isn’t affected by the tamper evident system of the case, despite there is evidence that it has already been opened. This type of solution is usually used for products of more than one usage.
- Without the possibility of closing afterwards. When designing these tamper evidence models, it is done so that the closure flaps of the case are disabled to close the packaging (they break or tear). Usually used in the case of single-use products and therefore, do not need to be stored again, such as, injectables.
This type of tamper-evident system has the advantage that it isn’t necessary to incorporate additional material into the packaging, for which it’s a more advantageous option than that of labels by cost-benefit criteria. However, changing the design of the flaps often leads to modifications in the packaging lines of pharmaceutical companies. Therefore, Nekicesa is in favor of designs that practically do not affect the equipment available in the lines. Our Innovation team is collaborating with the clients, and jointly developing proposals that allow the elaboration of solutions with the least impact for both parties.