If this is your company, CONTACT US to activate Packbase™ software to build your portal.
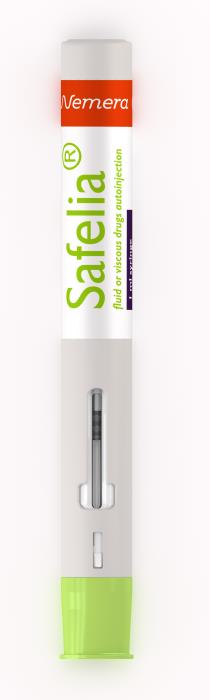

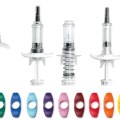
Nemera’s new generation 2-step autoinjector platforms are suitable for fluid to highly viscous injections. Safelia® has been designed to ease patient self-injection experience and to deliver even the most challenging drugs (viscous formulations up to several Centipoise, sustain released, concentrated, fragile and shear sensitive formulations, either in subcutaneous or intramuscular layers, on two platforms: up to 1ml or up to 2.25ml delivery).
Injectable formulations are the fastest growing segment in the pharmaceutical landscape. Biological therapies are increasingly used to treat a wide range of chronic diseases requiring frequent drug administration over a long period of time. Developing drug delivery devices able to administer the future pipeline of biological molecules is a challenge.
Biotherapeutics tend to be more viscous, concentrated and administered in larger volumes. The issue of patient adherence to their treatment is an additional challenge. Pharmaceutical companies tend to provide treatments with less frequent injections, more concentrated, with larger volumes to be injected. Autoinjector platforms have to evolve to adapt to these new parameters as well as integrating an optimized patient experience. Other key factors that can improve patients adherence to their injectable treatment are pain reduction, less bruising and shorter delivery times.
Nemera’s Safelia autoinjector platforms have been designed to respond to challenges of handling new formulations while taking patient needs into consideration. Safelia autoinjector platforms:
- Administer a large range of formulations and injection volumes; adapt to handle liquid injections to highly viscous formulations, taking specific care of the biologics; support sustained-released formulations delivery and shear sensitive molecules up to 2.25ml injection volumes
- Improve patient experience with the possibility to reduce needle gauge and injection time; slows down needle penetration inside body tissues; tailor-designs injection course to limit pressure peaks into body tissues and makes delayed retraction possible to allow enough time for body tissues to absorb the injected drug.
Nemera's Safelia autoinjector design is patented and includes the ability to handle high injection spring forces and deliver formulations in standard glass syringes. The autoinjector is not supported by the syringe’s flanges, which are structurally weak, but rather by the syringe's shoulder, allowing spring forces up to 70N with standard autoinjector plastic parts. If higher
viscosities are required to be delivered in less time, the autoinjector can be adapted with stiffer springs and adjusted materials.
The spring release shock and energy are absorbed by a rotating cam system (patented), and the energy is transmitted in compression and attenuated to the primary container. Risks of breakage are therefore reduced during the firing of the autoinjector but also during transportation or handling (dropping) of the device.
Safelia autoinjector platforms are available for customization in 1ml and 2.25ml versions, depending on the delivery specification of your formulation. Both IM and SQ designs are available. Examples of different tailored designs that adjust injection courses during delivery can be tested to better fit your formulations. Consult us for details.
Nemera has a well-know and established reputation in designing, developing and industrializing parenteral devices. As an example, every day over 5 million diabetics rely on devices manufactured by Nemera in our 4 manufacturing plants with harmonized high standard quality. Upstream of the production of pens, autoinjectors, and implanters, we rely on the expertise of our Innovation Centre of Development. Safelia development has benefited from the implication of creative design and human factor specialists, mechanical engineering, testing in our world-class Laboratory, manufacturing and assembly knowledge and extensive mathematical modeling.