Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.
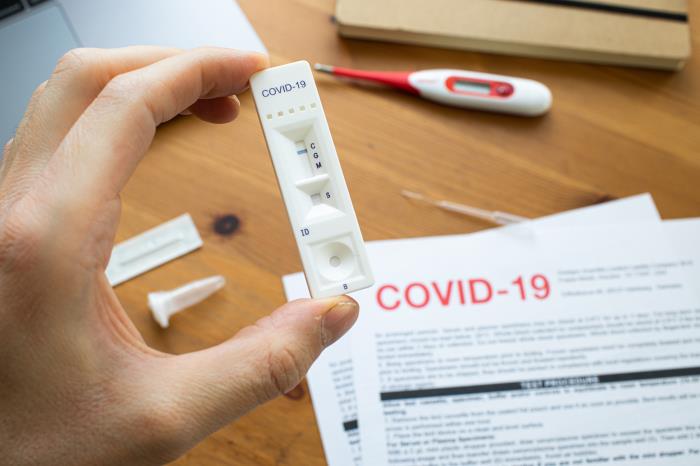

Aptar CSP Technologies,a leader in material science and active packaging solutions (part of AptarGroup, Inc.), announced its Activ-Film technology protects two new at-home COVID-19 tests that recently received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA).
The tests were developed by a leading manufacturer of diagnostic healthcare solutions and offer prescription and OTC (Over the Counter) COVID-19 at-home testing options without the need to visit a doctor’s office
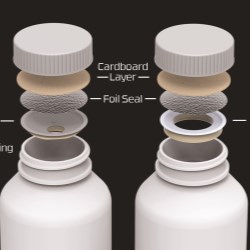
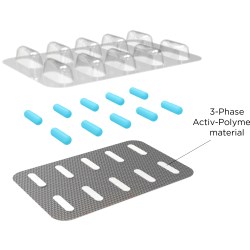
Aptar CSP’s Activ-Film technology is integrated into the dipstick of the diagnostic kits to protect against moisture and other environmental conditions that could otherwise impact test accuracy. Activ-Film leverages Aptar’s proprietary 3-Phase Activ-Polymer platform technology, which provides a broad spectrum of customized and highly-engineered solutions in a variety of configurations, such as Activ-Vial for housing diagnostics strips and dipsticks, and Activ-Tab, which is integrated within diagnostic cassettes.
This material science-based active packaging technology currently protects a range of lateral flow, molecular, and electrochemical diagnostic test kits on the market today, including Quidel Corporation’s QuickVue Influenza and COVID-19 tests.
“As we continue to navigate through the COVID-19 crisis, this game-changing solution will help meet the urgent demand for COVID-19 testing in communities around the world,” said Badre Hammond, Vice President Commercial Operations, Aptar CSP Technologies. “We are committed to leveraging our material science expertise to enable our partners to meet the ongoing need for innovative healthcare solutions that help improve and save lives. This is another example of the value we bring to the market when sensitive diagnostic elements need protection.”
More information about the new at-home COVID-19 tests is available on the FDA website.



.jpg)
.jpg)





.jpg)
.jpg)













.jpg)




.png)