If this is your company, CONTACT US to activate Packbase™ software to build your portal.
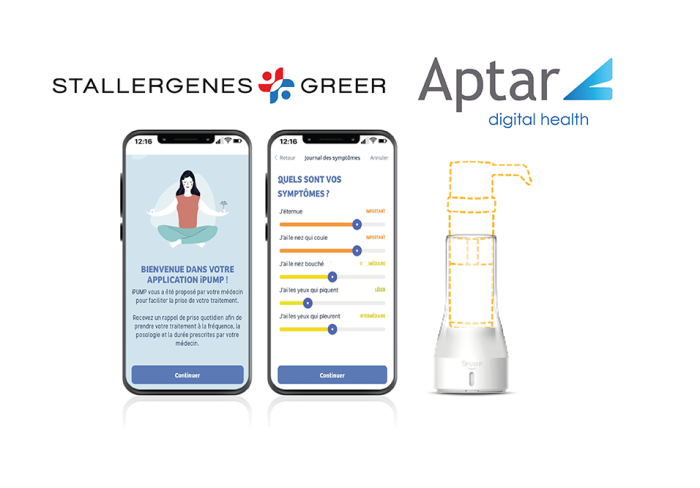

- iPUMP® is the first connected allergy management assistant, launched recently by Stallergenes Greer in France
Aptar Digital Health, a global expert in Software as a Medical Device (SaMD), digital Patient Support Programs (PSPs) and disease management solutions, has developed iPUMP®, the first connected allergy assistant which was recently launched in France by Stallergenes Greer, a global healthcare company specialising in Allergen Immunotherapy Treatment (AIT).
Designed for patients treated with Stallergenes Greer’s liquid sublingual AITs, the availability of iPUMP® followed a pilot phase conducted by Stallergenes Greer during which 92% of patients systematically used iPUMP® while taking their treatment. With the launch of this technological innovation, both Aptar Digital Health and Stallergenes Greer are responding to two of the major challenges in the day-to-day management of AIT treatments non-adherence and premature discontinuation of treatment.
“With iPUMP®, the patient experience in the management of AIT is simpler. Thanks to multiple functionalities, patients can closely monitor their adherence, thus optimizing their treatment outcomes,” said Sai Shankar, President of Aptar Digital Health. “This collaboration with a leading company in the field of AIT illustrates Aptar Digital Health’s ability to deliver innovative, effective, and easy-to-use solutions to improve the patient’s experience with their therapy.”
iPUMP® features a connected pressure sensor that converts the force exerted into an audible signal as soon as the threshold is reached, which confirms that the treatment has been taken. An integrated timer marks the end of the daily dose as prescribed, and a visual cue confirms if the daily dose has been taken.
iPUMP® is connected to a mobile application of the same name, which gives direct access to the protocol prescribed by the doctor. iPUMP® helps patients improve compliance with their treatment to optimize its efficacy while offering a function for tracking intake history for precise medication monitoring.
During the pilot phase, 74% of patients under 15 years of age adopted iPUMP® to improve their compliance, and almost two-thirds of participants perceived the solution as a real support in taking their liquid sublingual AIT.
This development and launch of iPUMP® marks an important milestone in a longstanding partnership between Aptar Digital Health and Stallergenes Greer. Based on their proprietary software platform, iPUMP® confirms Aptar Digital Health’s status as a major player in digital health innovation, as well as its capabilities to develop hardware and software solutions to the benefit of both the patient and the medical community.


.jpg)



.jpg)
.png)
.jpg)



 copy.jpg)


.jpg)
.jpg)


























.jpg)


