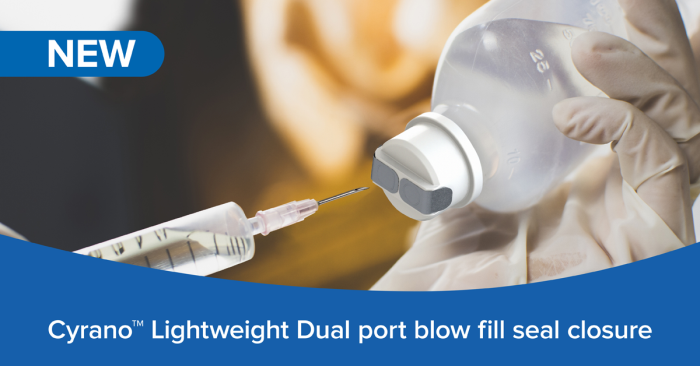

Berry Global Healthcare is launching what the company claims is the lightest dual-port blow fill seal closure currently available for large volume parenteral applications – up to 64% lighter than some competitor products. The new lightweight Cyrano provides an important sustainability benefit through the reduction in overall plastic usage while offering a safe and easy-to-use solution for all types of intravenous and infusion systems in hospitals and other clinical environments.
Cyrano uses Berry’s advanced molding technology to create extremely lightweight components that still deliver a reliable performance to safeguard product integrity, ease drug administration, and improve patient safety. The standard ready to use closure is validated for use on the latest blow-fill-seal machines and is certified to ISO 15 759.
Cyrano’s twin in and out port design is sealed with a protective aluminum foil, offering a flat surface for fast and easy disinfection. The swift peel-off action of the foil also saves time during administration. Importantly, the foil protects each port individually, meaning one port can be opened for access while the other remains closed and sterile.
In addition, the two separate ports also offer maximum flexibility during usage, for example allowing individual sides for injection and infusion.
The silicone and rubber-free closure provides superior seal integrity for effective protection against drug spillage, tampering, microbial, chemical, and particle contamination. In addition, it offers excellent spike retention, automatically resealing after being pierced.
The bi-injected Cyrano is available in both medical-grade polypropylene and polyethylene to ensure maximum compatibility during filling, sterilisation, and sealing. The closure can be incorporated into blow fill seal equipment lines, with excellent welding ability for a secure fit with the bottle. The foil can be printed to enable branding and personalisation of the closure.
Muriel Combeau, Sales and Marketing Director, Berry Global Healthcare, commented:
“By removing unnecessary weight and using fewer plastic elements in its manufacture, we have developed a closure that offers a valuable sustainability benefit with no compromise on performance.
Cyrano is a highly flexible, and also cost-efficient, solution for many types of parenteral requirements and the administering of preparations by IV or infusion. In particular, its dual port design allows greater adaptability and ease of use compared to more traditional twin cap versions.”




























.jpg)
















