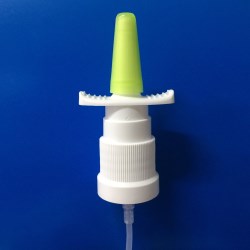
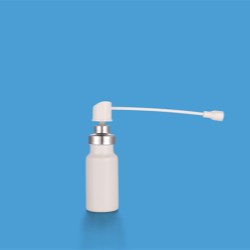
Single dose Dry Powder Inhalers that work with medicinal capsules. Designed to be comfortable and easy-to-use by the end user for the treatment of COPD and asthma. Manufactured from medical ABS, all materials comply with CP, EP and USP food and drug direct contact regulations. Custom design and development is available upon request.
Bona Pharma's Dry Powder Inhalers are ISO15378 certified and benefit from CE and US FDA approval.
Overview | |
| Reference - Main | 2 |
|---|---|
| Reference - Sub | BONA20NDPI |
| Packaging Type | Pharmaceutical Components |
| Packaging Sub-Type | Oral Inhalers |
| Market - Major | Health |
| Market - Segment | Pharmaceuticals |
| Market - End Use | OTC (Over the Counter) Drugs. Prescription Drugs. Illness Prevention |
| Certifications | Laboratory, Testing, Certification. ISO Certifications. ISO 15378 - Medicinal Packaging Materials. FDA Approved. CE Mark |
Characteristics | |
| Materials | Plastic. Plastic - ABS |
|---|---|
| Solution Type |
|
Dimensions | |
| Width | 21.4 mm |
|---|---|
| Length | 39.8 mm |
| Height | 68.8 mm |
| Weight | 13.6 g |
Neck | |
| Neck Diameter | 18 mm |
|---|---|