If this is your company, CONTACT US to activate Packbase™ software to build your portal.


Clariant Plastics & Coatings Healthcare Polymer Solutions recently launched static-reducing functional plastic compounds which, when used in drug delivery devices, enable increased dose reliability. The resins are part of the wider ‘medical grade’ MEVOPUR® line of color and additive concentrates and ‘ready-to-use’ polymer compounds, covering polymers from PE to PEEK.
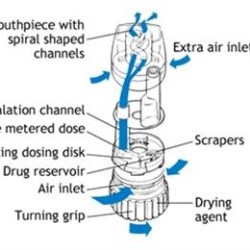
Plastics are insulators, and therefore prone to build-up of static charges. In applications where plastics are in contact with powders, the powder can stick to the surface. In drug delivery devices, such as dry powder inhalers, this sticking effect reduces the dose reliability and repeatability. MEVOPUR permanent antistatic compounds reduce the surface resistivity and dissipate charges quickly, thereby eliminating sticking. However, since the plastics often have direct drug contact, and inhalation is considered as a medium-to-high risk drug administration route, the plastic materials need to be evaluated for biocompatibility and risk of potential leachables.
In a recent development program with a leading European pharmaceutical company, members of Clariant’s global Healthcare Polymer Solutions team provided technical support and compounded materials covering ABS, COP (cyclic olefin polymer) and PP resins that the development team planned to use. The critical areas in contact with the drug flow needed to avoid static charge build- up, and the MEVOPUR antistatic agent, combined with the chosen polymer, demonstrated antistatic functionality that significantly reduced this problem. In other development programs, antistatic functionality was combined with the chosen color in one compound product.
Although many resins are now widely available as ‘medical grade’ products with supporting regulatory declarations and change notification, this usually only covers the resin, and not colors or additives that are introduced by compounding or masterbatch concentrates. It is these colors and additives that present the higher risk of potential leachables that could end up in the drug. The problem is particularly acute as these added materials change routinely over the life time of the product.
The MEVOPUR family significantly reduces this risk by using pre-tested ingredients that support compliance to medical and pharmaceutical standards such as ISO10993 and USP Class VI, manufactured in EN- ISO13485:2016 certified production facilities and change control.
Other examples where MEVOPUR anti-static compounds are applied in drug delivery is in transparent plastic ‘spacer’ accessories used with metered-dose inhalers (MDI). These spacers which act as ‘accumulators’ to aid in correct dosing, are mainly used by very young or elderly patients who have difficulty to use an MDI unaided. Although anti-static functionality is needed, the materials used are often not ‘medical grade’, and so they can present the same risk as the described above. MEVOPUR antistatic compounds are available both in opaque colors and in transparent MABS (methyl methacrylate-acrylonitrile-butadiene-styrene) delivering the same quality and regulatory compliance.
“Recently, we introduced the theme of ‘The Color of Innovation” to show how early collaboration with Clariant’s expert team and applying QbD (Quality by Design) principles to material decisions, widen the scope of possibilities,” explains Steve Duckworth, Global Head of Marketing & Business Development- Healthcare. ‘This is about bringing new ideas and capabilities to designers, but without sacrificing on regulatory compliance. This is why we say that MEVOPUR helps make every decision an opportunity.”