Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.

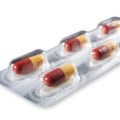
CSP Technologies, Inc., a leader in packaging solutions that ensure product protection, enhance brand recognition and improve consumer experiences, introduced Activ-Blister™ solutions, an innovative technology that protects moisture- and oxygen-sensitive solid dose pharmaceuticals, at INTERPHEX NYC, Booth #2059, April 26-28 at the Jacob Javits Center.
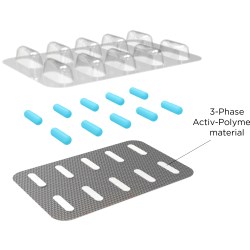
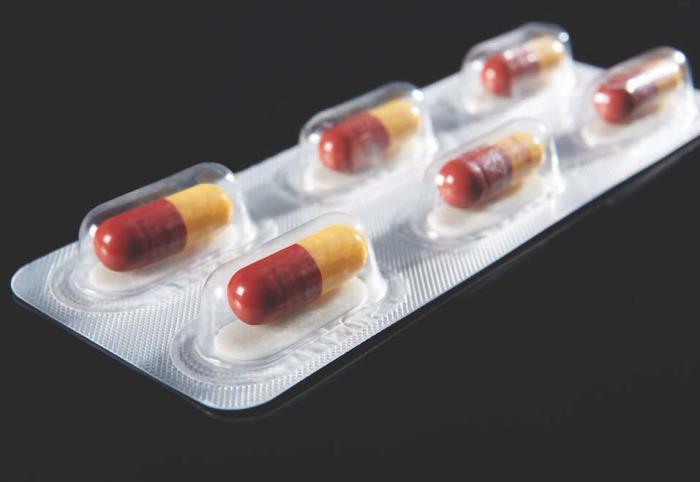
CSP Activ-Blister materials from CSP Technologies protect moisture- and oxygen-sensitive solid doses packaged on thermoform-fill- seal and fill-seal equipment. Using silica gel and molecular sieve technology, outfitted blisters can absorb tailored amounts of water vapor, oxygen, or a combination of the two, and can be produced in shapes and sizes to accommodate any tablet and capsule size.
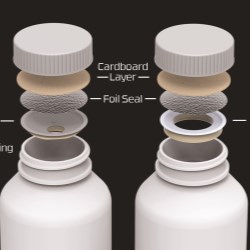
CSP Technologies developed Activ-Blister solutions to control the internal atmosphere of existing individual blister cavities, allowing for improved product performance and enhanced shelf-life. Offering moisture, oxygen, and combination absorption, the technology can be applied without the use of adhesives and without changes to the existing footprint of a packaging line. Activ-Blister solutions can be incorporated into a wide range of blister packaging formats, including push-through blisters, peel/push blisters, cold-form foils and high barrier films containing Aclar ® laminates.
For pharmaceutical manufacturers and contract packagers, Activ-Blister solutions, a patented technology, enables pharmaceutical manufacturers and packagers to achieve premium moisture protection without automatically resorting to the use of expensive cold-form foils. Eliminating the need for cold-form foils also allows for a smaller blister footprint, up to 40-60% size reduction, and provides clear visibility of the tablet/capsule in the blister cavity. Additionally, products that are normally packaged in bottles with desiccant sachets can now be thermoformed into blister cards without sacrificing moisture protection. This move from bottle to blister effectively eliminates the added costs associated with gas flush/purge and a secondary packaging with sachets.
“By integrating precise desiccant and/or gas-absorption into the individual blister with adhesive- free technology, products are protected against moisture, oxygen, and odors,” said Craig Voellmicke, Vice President Business Development, CSP Technologies, Inc. “In addition, products enjoy enhanced shelf life when compared to cold-form foil and standard thermoform solutions.”
CSP Technologies has decades of experience in providing custom polymeric and packaging solutions for companies around the world and currently has the leading global Activ-Vial ® portfolio for pharmaceuticals and diagnostics applications. According to Voellmicke,” The company delivers outstanding service along with innovative products that are specifically engineered to meet customers’ exact product challenges.”



.jpg)
.jpg)





.jpg)
.jpg)













.jpg)




.png)