Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.
.jpg)

Sophisticated implantable medical device for urinary and bowel dysfunction leverages Aptar’s groundbreaking 3-Phase Activ-Polymer technology to control moisture and extend use life.
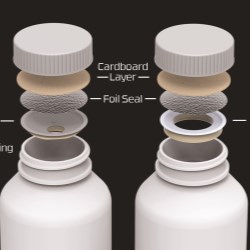
Aptar CSP Technologies, a leader in active material science solutions (part of AptarGroup, Inc.), announced that its proprietary Activ-Film technology was approved by the U.S. Food and Drug Administration (FDA) for use within a recently launched Rechargeable Implantable Neurostimulator (INS) that treats urinary and bowel dysfunction. The Activ-Film material is integrated into the medical device to control humidity, improve the accuracy of readings, and extend use life.
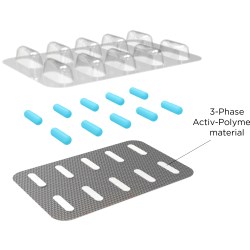
Aptar CSP’s Activ-Film material leverages the company’s patented 3-Phase Activ-Polymer platform technology to manage the device’s internal atmosphere, adsorbing moisture that could otherwise accumulate and affect the implant’s stability and performance. Controlling the humidity within the device protects the battery and enables it to have little to no degradation during its use life.
“This is another example of our consistent commitment to helping customers bring advanced solutions to patients and improve user experiences and outcomes,” said Badre Hammond, Vice President Commercial Operations, Aptar CSP Technologies. “We are dedicated to continued material science innovation that facilitates the development and commercialization of next generation implantable medical devices.”
Activ-Film material is a configuration of Aptar CSP’s 3-Phase Activ-Polymer technology. In addition to protecting a range of implantable medical devices, the highly-engineered technology is utilized in various formats for a broad array of applications, including oral solid dose drugs, drug delivery systems and probiotics. The material science technology can be customized to deliver integrated active solutions that absorb moisture, scavenge oxygen, odor, or VOCs, emit aromas, or reduce pathogens.



.jpg)





.jpg)
.jpg)













.jpg)




.png)