Public
Aptar CSP News
Aptar CSP Technologies Documents
Aptar CSP Technologies Locations
Aptar CSP Technologies Videos
Subsidiaries
Maxwell Chase Technologies
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


Activ-Blister™ active packaging solutions deliver enhanced product stability and expedited speed-to-market for oral solid dose drugs in the EU
Aptar CSP Technologies, part of AptarGroup, Inc. and a leader in active material science solutions to ensure product protection, extend shelf life and improve patient experience, announces a new manufacturing site able to produce its Activ-Blister™ Solutions for oral slide dose drugs in Europe. The move is part of a key strategic effort to expand production of Activ-Blister™ technology globally and is the result of a 3-way collaboration between Aptar CSP, Uhlmann (a leading pharmaceutical packaging equipment manufacturer), and Ivers-Lee, www.iverslee.com, (a local contract manufacturing organization).
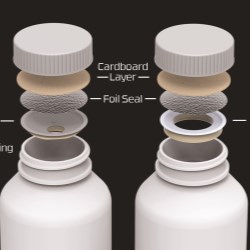
This collaboration consists of a fully automated Uhlmann blister machine validated to package oral solid dose drug products with Activ-Blister™ Solutions heat-staked to the foil at a rate of up to 250 blisters per minute (Figure 1). The equipment is designed to integrate Activ-Blister™ technology into the blister cavity with precision placement (Figure 2). The equipment has been fully-validated and is in place at Ivers-Lee, a CMO capable of manufacturing Activ-Blister™ solutions from R&D to commercialization. A CMO in EMEA will support regional customers and ensure speed-to-market.
“Expanding global manufacturing sites for Activ-Blister™ technology reflects our commitment to delivering global access to innovative active material science solutions for patients and consumers,” said Badre Hammond, VP Commercial Operations and GM APAC, Aptar CSP Technologies. “With manufacturing capabilities now available in in both the US and EMEA, we will turn our attention to bringing production of this technology to the Asia market.”
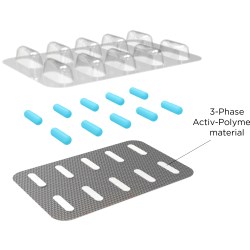
Activ-Blister™ Solutions integrates Aptar CSP’s proprietary 3-Phase Activ-Polymer™ platform technology into individual blister cavities to provide precision microclimate protection for sensitive drug products. This technology can be customized specifically for the drug developer’s formulation to provide a broad spectrum of specific drug protection including moisture adsorption, and oxygen and odor scavenging. The technology can also scavenge volatile organic compounds (VOCs) and emit aromas.
Representatives from Aptar CSP Technologies will be available at Pharmapack on May 18-19 in Paris, France to provide more information on this collaboration and the opportunities it presents. Visit Aptar at booth E46 or contact Francois Bidet at +33 6 2078 8153 or Francois.Bidet@Aptar.com to schedule a meeting.
Figure 1: Uhlmann Blister Machine Used to Manufacture Activ-Blister™ Solutions at Ivers-Lee
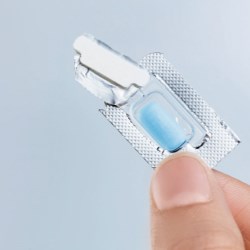
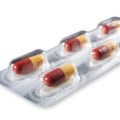
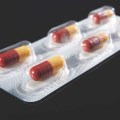
Figure 2: Activ-Blister™ technology remains attached to foil backing when blister is opened for enhanced patient safety




.jpg)
.jpg)





.jpg)
.jpg)













.jpg)




.png)