Public
Gerresheimer Brochures
Gerresheimer Gallery
Gerresheimer Locations
Gerresheimer News
Gerresheimer Product Catalog
Gerresheimer Publications "Update"
Gerresheimer Videos
Subsidiaries
Sensile Medical
If this is your company, CONTACT US to activate Packbase™ software to build your portal.
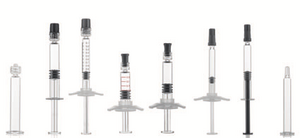

Biopharmaceuticals demonstrate a series of special features. They are often highly viscous and in individual cases tend to interact with silicone oil or, for example, tungsten residue from syringe production. Gerresheimer therefore offers integrated thin-walled cannulas for the improved emptying capability of the syringe for these applications. The special silicone treatment for the reduction of free silicone oil (baked-on) and the "metal-free" production without tungsten pin create a perfect primary packaging material for these sophisticated medications.
Metal-free syringe
A problem when using syringes can be traces of tungsten or other metals that occasionally remain behind when shaping the syringe cone in the bore. Especially for medications based on biotechnologically manufactured active ingredients, our customers therefore require pre-fillable syringes that ideally exclude the possibility of contamination with metal. With the development of an innovative production technology registered for patent, we have been able to address this wish and create a metal-free 1 ml long Luer Lock Gx RTF syringe that is ready for series production. A transfer to other Luer Lock syringe sizes or to Luer cone syringes of various sizes is possible at all times.
The pin used to shape the cone with the new technology is no longer made from the tungsten usually used or an alternative metal, but instead of a special ceramic. External tests show that the new process for shaping the cone works free of residue. This means that not only traces of metal, but also contamination through the ceramics used are excluded. The Institut Fresenius neither found ceramic particles nor detected tissue reactions in a separate bio-compatibility study.
Baked-on siliconization for the reduction of free silicone oil
Pre-fillable syringes are very often used for the storage and administering of biotechnological medications. They are generally siliconized in order to ensure the lowest possible and most constant forces possible for the storage duration when using the syringe. The siliconization also hydrophobizes the glass surface and in this way ensures the complete emptying capability for the medication and a low level of interaction of the active ingredient with the glass. However, high silicone quantities result in the formation of the smallest silicone droplets in the solution, onto which the active ingredient can coagulate and is then no longer bioavailable.
With our Gx Baked-on RTF syringes patented in Europe and the USA, Gerresheimer has therefore developed a technology that achieves gap-free coating with the lowest quantities of silicone possible. In this process, a silicone oil emulsion is sprayed into the syringe body and subsequently affixed to the surface by heating. Despite the considerably reduced amount of silicone used and the correspondingly minimized load of free silicone droplets, the syringes provide a reliable smooth coating, as well as stable breakaway and sliding forces over the entire storage period. Gerresheimer can also equip the syringes with thin-walled cannulas that ease the administering of the often highly viscous medications thanks to their improved flow properties.



.jpg)
.jpg)
.jpg)













.jpg)