Public
Nordson EFD Catalog
Nordson EFD Catalog for Animal Health Solutions
Nordson EFD Certificates
Nordson EFD Links
Nordson EFD Locations
Nordson EFD News
Nordson EFD Videos
If this is your company, CONTACT US to activate Packbase™ software to build your portal.


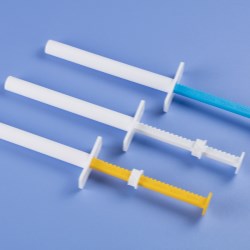
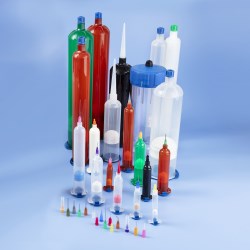
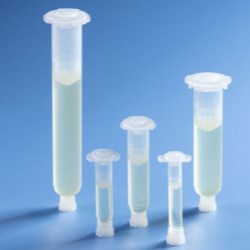
Using USP Class VI certified resins to mold a new line of fluid dispensing components, Nordson EFD provides assurance of the quality and safety of its products to manufacture medical devices.
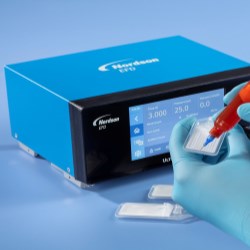
Nordson EFD, a Nordson company (NASDAQ: NDSN), the world’s leading precision fluid dispensing systems manufacturer, introduces Optimum® Class VI fluid dispensing components made from 100% virgin resin that is Class VI certified by the U.S. Pharmacopeial Convention (USP).
“We wanted to make it easier for medical manufacturers to provide the traceability required to meet strict regulatory standards,” said Felicitas Stuebing, global product line specialist at Nordson EFD. “Meeting the most stringent USP Class designation is not only a testament to the quality of our components, it provides peace of mind that our components are safe to use in medical manufacturing processes.”
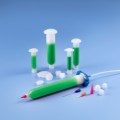
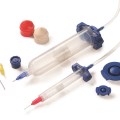
New Optimum Class VI dispensing components include 3cc, 5cc, 10cc, 30cc, and 55cc syringe barrels and pistons, in addition to end caps and tip caps. These biocompatible components do not have a colorant, making them easier for engineers to document for regulatory approvals.
In addition, these first-to-market medical-grade dispensing components feature the same highly-engineered design as standard Optimum components. The Class VI syringe barrels feature ZeroDraft™ walls with consistent internal diameters that maintain an airtight seal with Class VI pistons to provide accurate, repeatable fluid deposits used in bonding, coating, and other medical assembly processes.
These components also pass the same rigorous quality standards Nordson EFD maintains to ensure a consistent product that provides consistent performance.
Ethylene oxide (ETO), autoclave, and gamma sterilization methods were tested to determine the impact on Optimum Class VI dispensing components. Those results can be found in the product data sheet, though Nordson EFD states that all customers must do their own testing to validate their processes.