If this is your company, CONTACT US to activate Packbase™ software to build your portal.
.jpg)

Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, has collaborated with AARDEX Group, an expert in digital medication adherence, to offer electronic solutions for medication adherence monitoring during clinical trials. The new offerings enhance therapy adherence in clinical trials, enabling pharmaceutical companies to digitally monitor and manage medication intake of trial participants.
During clinical trials for new drugs, pharmaceutical companies must be able to depend on strict adherence to therapy regimens. However, up to 50 percent of patients involved in clinical trials do not adhere to the prescribed dosage regimen, resulting in distorted outcomes such as insufficient drug efficacy or underestimated frequency of side effects. Ultimately, these adverse events can delay time to market.
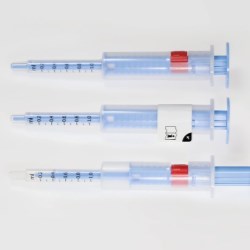
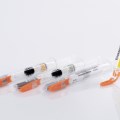
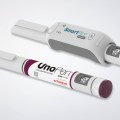
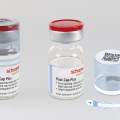
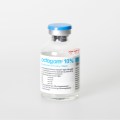
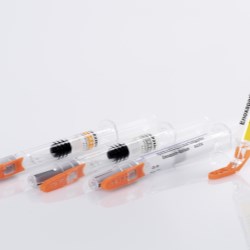
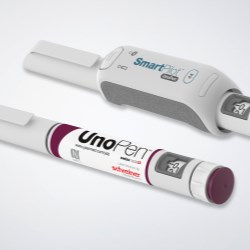
The therapy monitoring solution consists of smart medicine packaging linked to matching software. Schreiner MediPharm has developed Smart Blister Packs for tablets and capsules and Smart Kit Boxes for vials and syringes with sensor technology for this solution: When a patient extracts a tablet from a cavity or removes a vial from a compartment, real-time data are generated such as time of removal, compartment from which the product is extracted, and dose. This information is automatically stored and transmitted to a database via a smartphone app or reader.























.jpg)